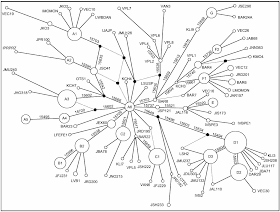
Last year I published a post on Networks of affinity rather than genealogy, in which I listed the publications from 1750-1900 that I know contain affinity networks. These are non-directional networks showing affinity among taxa, rather than showing genealogical relationships among the taxa.
Affinity refers to a natural (rather than artificial) overall group resemblance, usually quantified (in modern terminology) by some sort of weighted similarity of characters. Patterns of affinity may, indeed, result from evolutionary relationships, but affinity is a much broader concept than genealogy — in particular, affinity relationships are usually multi-directional rather than nested. This distinction between affinity and genealogy runs throughout the history of depictions of biological relationships, and continues to this day.
The importance that I see in these historical networks is that they match closely the modern idea of unrooted data-display networks. In this post I update my list of affinity networks, and add some more notes about their origins.
Maps versus networks
The important point here is that affinity is usually imagined as being multi-facetted, so that any diagram of affinities shows multiple connections among the taxa, and relationships between groups are very definitively reticulating. However, one point that I did not sufficiently emphasize in the previous blog post is that there are all sorts of other representations of reticulate relationships that have been used by biologists, some intended to be solid figures with faces, others are interlocking circles or radiating hexagons or nested ovals, and some have been explicitly referred to as maps. There is also what are referred to as quinarian classifications, in which taxa are arranged in groups of five that show multiple relationships.
Most of these diagrams could be converted to a network graphical representation. However, a network diagram should strictly have relationships indicated solely by connecting lines, rather than by overlapping circles, etc. That is, we can distinguish between networks in the strict sense and what are usually called "maps" in the broad sense. The latter name comes from Carl von Linné (see the quotation below). So, both networks and maps show reticulate relationships, but networks are still connected by lines even when any enclosing structure of groups is deleted.
I have used this strict definition of networks to update my list of affinity networks. This differs from the list in the previous post mainly by deleting two publications (which are better considered as maps not networks) and adds two more networks.
Affinity networks
Here is a list of the publications from 1750-1900 containing affinity networks that I know about. I have indicated my source, and I have also linked to an online copy of the diagram. (I have extracted some of the figures from Gallica, Google Books or the Biodiversity Heritage Library, where they are otherwise unavailable online.)
- 1774 Johann Philipp Rühling "Ordines Naturales Plantarum Commentatio Botanica" [Ragan Fig. 7, Stevens Fig. 12, Barsanti Fig. 26, Pietsch Fig. 18]
- 1777 Johann Hermann "Tabula affinitatum animalium" [Barsanti Fig. 31] republished in 1783 [Ragan Fig. 8]
- 1802 August Johann Georg Carl Batsch "Tabula Affinitatum Regni Vegetabilis" [Ragan Fig. 9, Barsanti Fig. 43, Pietsch Fig. 19]
- 1825 Adrien-Henri-Laurent de Jussieu "Sur le groupe des Rutacées." Mémoires du Muséum d'Histoire Naturelle 12: 384-542 [Ragan Fig. 13, Stevens Fig. 10, Pietsch Fig. 30]
- 1826 Leopold Joseph Franz Johann Fitzinger "Neue Classification der Reptilien" [Gaffney Fig. 2]
- 1841 Eduard Fenzl "Darstellung und Erläuterung vier minder bekannter, ihrer Stellung im natürlichen Systeme nach bisher zweifelhaft gebliebener, Pflanzen-Gattungen." Denkschriften der Königlich-Baierischen Botanischen Gesellschaft zu Regensburg 3: 153-270 [Stevens, figure]
- 1843 Adrien-Henri-Laurent de Jussieu "Monographie de la famille des Malpighiacées." Archives du Muséum d'Histoire Naturelle 3: 5-151 [Stevens, figure]
- 1844 Henri Milne-Edwards "Considérations sur quelques principes relatifs à la classification naturelle des animaux et plus particulièrement sur la distribution méthodique des mammifères." Annales des Sciences Naturelles, Sér 3 Zoologique 1: 65-99 [Barsanti Fig. 59, figure]
- 1872 Alexander Andrejewitch von Bunge "Die gattung Acantholimon Boiss." Mémoires du Academie Imperiale des Sciences de St Pétersbourg Série 7 18(2): 1-72 [Stevens Fig. 19]
- 1888 Ferdinand Albin Pax "Monographische übersicht über die arten der gattung Primula." Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 10: 75-241 [Stevens, there are 15 figures]
- 1889 Julien Vesque "Epharmosis, sive Materiae ad Instruendam Anatomiam Systematis Naturalis. Pars Prima. Folia Capparearum" [Stevens, figure]
- 1889 Julien Vesque "Epharmosis, sive Materiae ad Instruendam Anatomiam Systematis Naturalis. Pars Secunda. Genitalia Foliaque Garciniearum et Calophyllearum" [Stevens, figure]
- 1890 Franz Georg Philipp Buchenau "Monographia Juncacearum." Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie 12: 1-495 [Stevens, figure]
- 1893 Georg Klebs "Flagellatenstudien, Theil II." Zeitschrift für Wissenschaftliche Zoologie 55: 353-445 [Ragan Fig. 26]
- 1895 Olga Tchouproff "Quelques notes sur l'anatomie systématique des Acanthacées." Bulletin de l'Herbier Boissier 3: 550-560 [Stevens, figure]
- 1896 Nicolai Ivanovich Kusnezov "Subgenus Eugentiana Kusnez. generis Gentiana Tournef." Acta Horti Petropolitani 15: 1-507 [Ragan, Stevens Fig. 18, Pietsch Fig. 91]
- 1898 Émile Constant Perrot "Anatomie comparée des Gentianacées." Annales des Sciences Naturelles: Botanique, Série 8, 7: 105-292 [Stevens, figure]
Some historical notes
Other early authors described biological relationships as being like a reticulating network, without any necessarily genealogical interpretation, even though they apparently did not themselves produce network diagrams. (It seems that only 5 of the first 11 historical references to network relationships provided a diagram.) Here are some known examples (with source):
- 1750 Vitaliano Donati "Della Storia Naturale Marina dell' Adriatico" [Ragan]
- 1792 Giuseppe Olivi "Zoologia Adriatica" [Ragan]
- 1802 Gottfried Reinhold Treviranus "Biologie, oder Philosophie der Lebenden Natur für Naturforscher und Aerzte, Band 1" [Ragan]
- 1824 Johann Heinrich Friedrich Link "Elementa Philosophiae Botanicae" [Stevens]
- 1828 Georges Cuvier "Histoire Naturelle des Poissons, Tome Premier" [Ragan]
- 1836 Constantin Rafinesque "Flora Telluriana" [Stevens]
In addition, the links of the chain are joined in such a way with the links of another chain, that the natural progressions should have to be compared more to a net than to a chain, that net being, so to speak, woven with various threads which show, between them, changing communications, connections, and unions.” [Translated from the Italian by Ragan 2009]This was followed immediately by a very similar idea from Carl Linnaeus (1751 "Philosophia Botanica"), who also did not provide a diagram:
Aphorism 77: All plants show affinities on either side, like territories in a geographical map. [Translated from the Latin]The first figure of relationships drawn as a map was apparently Linnaeus' own map of plant families [see Barsanti Fig. 37, Pietsch Fig. 16], published by two of his former students, J.C. Fabricius and P.D. Giseke, in a posthumous collection of his lectures (Giseke 1792 "Caroli a Linné Praelectiones in Ordines Naturales Plantarum").
Modern phylogenetics has used a tree as the preferred means of depicting relationships, rather than a network or map. The tree metaphor seems to have first come from Peter Simon Pallas (1766 "Elenchus Zoophytorum"), who explicitly acknowledged the earlier ideas:
As Donati has already judiciously observed, the works of Nature are not connected in series in a Scale, but cohere in a Net. On the other hand, the whole system of organic bodies may be well represented by the likeness of a tree that immediately from the root divides both the simplest plants and animals, [which remain] variously contiguous as they advance up the trunk, Animals and Vegetables. [Translated from the Latin by Ragan 2009]Note that the tree metaphor was explicitly intended by Pallas to be a simplification of the previously proposed network metaphor.
Actually, reticulating diagrams dominated over over trees in the literature until the publication of Charles Robert Darwin's major work (1859 "On the Origin of Species"). Darwin had two effects that are important for the discussion of metaphors.
First, he replaced the idea of an inherent order with a less ordered view of biodiversity as resulting from the contingencies of natural selection. This meant that the previous need for metaphors that allowed for multiple relationships among taxa (required to express the observed complexity of biodiversity), and hence the documented preference for reticulating diagrams (networks, maps, circles, cones, etc) was no longer needed. Darwin focused attention solely on genealogical relationships, to the exclusion of all others.
Second, Darwin championed the tree as the appropriate metaphor. This was possible because descent with modification can easily be expressed in a tree, provided that we focus (as he did) on vertical genealogical relationships (ancestor–descendant) rather than horizontal ones. One of his most famous quotes is:
The affinities of all the beings of the same class have sometimes been represented by a great tree ... The green and budding twigs may represent existing species), and those produced during each former year may represent the long succession of extinct species.Darwin knew about horizontal evolutionary events like hybridization, but he did not really integrate them into his metaphor. Darwin did not use the word "network" but he did use the word "web" with regard to affinity:
We can clearly see how it is that all living and extinct forms can be grouped together in one great system), and how the several members of each class are connected together by the most complex and radiating lines of affinities. We shall never, probably, disentangle the inextricable web of affinities between the members of any one class.
Barsanti G. (1992) La Scala, la Mappa, l'Albero: Immagini e Classificazioni della Natura fra Sei e Ottocento. Sansoni Editore, Firenze.
Gaffney E.S. (1984) Historical analysis of theories of chelonian relationship. Systematic Zoology 33: 283-301.
Pietsch T.W. (2012) Trees of Life: a Visual History of Evolution. Johns Hopkins Uni. Press, Baltimore.
Ragan M. (2009) Trees and networks before and after Darwin. Biology Direct 4: 43.
Stevens P.F. (1994) The Development of Biological Systematics: Antoine-Laurent de Jussieu, Nature, and the Natural System. Columbia Uni. Press, New York.